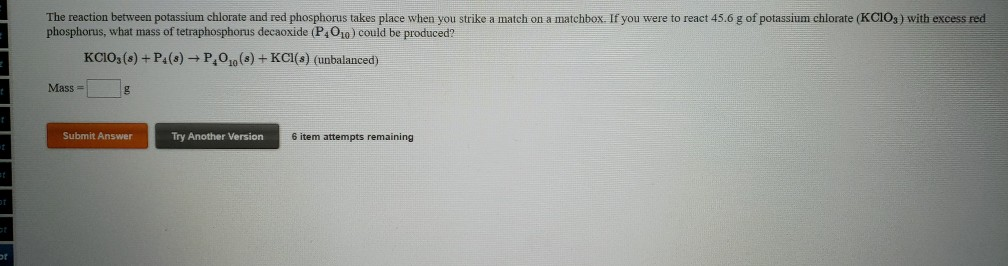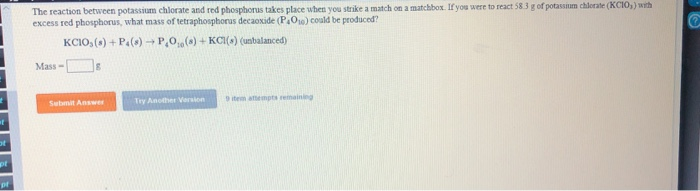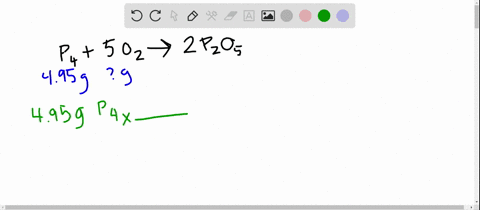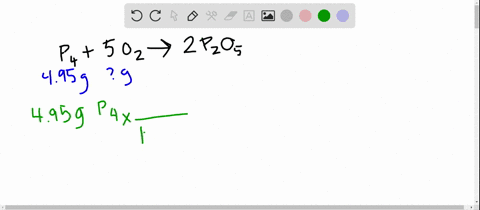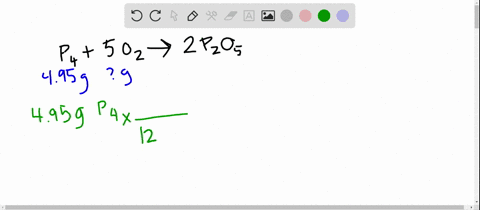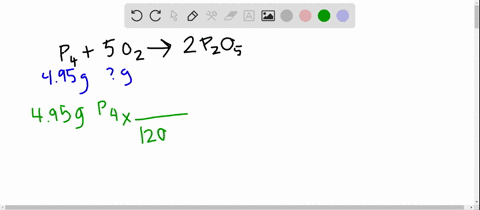
SOLVED:The reaction between potassium chlorate and red phosphorus takes place when you strike a match on a matchbox. If you were to react 52.9 g of potassium chlorate \left(\mathrm{KClO}_{3}\right) with excess red

Q 43- White phosphorus ismore dangerous thanAntimony trisulphidePotassium chlorateRed phosphorusAll of these | Snapsolve

SOLVED:The reaction between potassium chlorate and red phosphorus takes place when you strike a match on a matchbox. If you were to react 52.9 g of potassium chlorate \left(\mathrm{KClO}_{3}\right) with excess red

SOLVED:The reaction between potassium chlorate and red phosphorus takes place when you strike a match on a matchbox. If you were to react 52.9 g of potassium chlorate \left(\mathrm{KClO}_{3}\right) with excess red

SOLVED:The reaction between potassium chlorate and red phosphorus takes place when you strike a match on a matchbox. If you were to react 52.9 \mathrm{g} of potassium chlorate \left(\mathrm{KClO}_{3}\right) with excess red