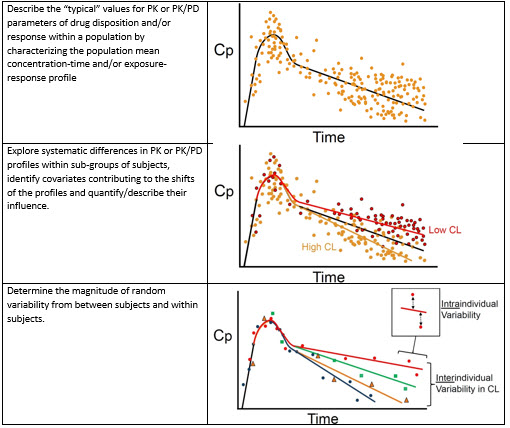
Overview of PopPK analysis. IIV, inter-individual variability; PopPK,... | Download Scientific Diagram

Model-Informed Drug Development, Pharmacokinetic/Pharmacodynamic Cutoff Value Determination, and Antibacterial Efficacy of Benapenem against Enterobacteriaceae | Antimicrobial Agents and Chemotherapy
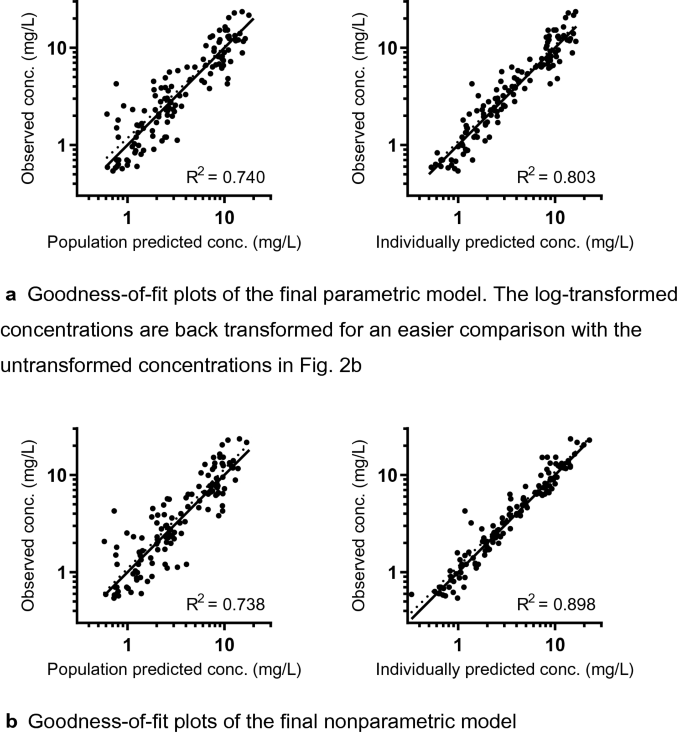
Population Pharmacokinetics of Imipenem in Critically Ill Patients: A Parametric and Nonparametric Model Converge on CKD-EPI Estimated Glomerular Filtration Rate as an Impactful Covariate | SpringerLink
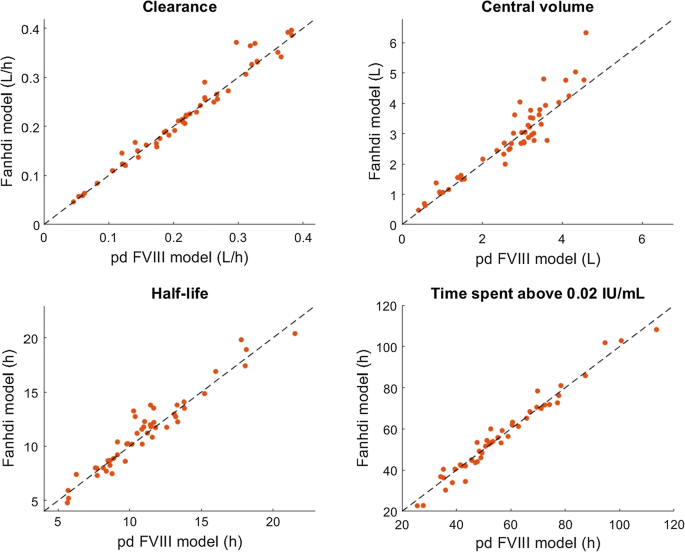
Routine clinical care data for population pharmacokinetic modeling: the case for Fanhdi/Alphanate in hemophilia A patients | SpringerLink

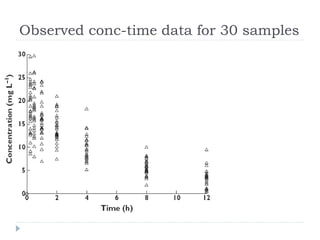
PopPK analysis: prediction-corrected visual predictive check for (a)... | Download Scientific Diagram

Pharmaceutics | Free Full-Text | Combining Therapeutic Drug Monitoring and Pharmacokinetic Modelling Deconvolutes Physiological and Environmental Sources of Variability in Clozapine Exposure | HTML

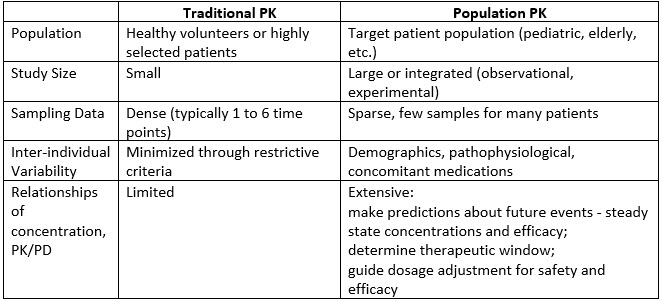
Establishing Best Practices and Guidance in Population Modeling: An Experience With an Internal Population Pharmacokinetic Analysis Guidance - Byon - 2013 - CPT: Pharmacometrics & Systems Pharmacology - Wiley Online Library

Clinical applications of population pharmacokinetic models of antibiotics: Challenges and perspectives - ScienceDirect

Population Pharmacokinetics Analysis of Quetiapine Extended-release Formulation in Japanese Patients with Bipolar Depression - Clinical Therapeutics

Food Effect Projections via Physiologically Based Pharmacokinetic Modeling: Predictive Case Studies - Journal of Pharmaceutical Sciences
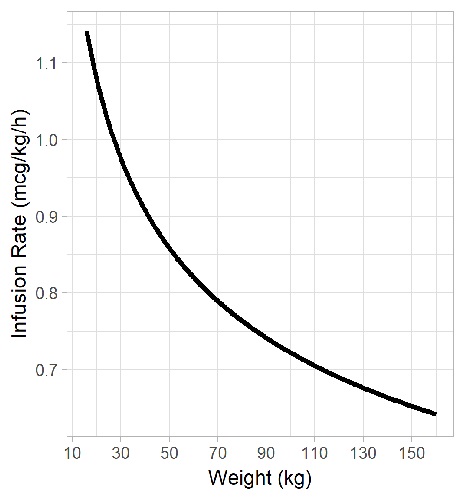
Dosing of Continuous Fentanyl Infusions in Obese Children: A Population Pharmacokinetic Analysis | PKMS Lab | UBC

Development of a population pharmacokinetic model of olanzapine for Chinese health volunteers and patients with schizophrenia | BMJ Open
Bayesian Population Physiologically-Based Pharmacokinetic (PBPK) Approach for a Physiologically Realistic Characterization of Interindividual Variability in Clinically Relevant Populations

Development of a population pharmacokinetic model of olanzapine for Chinese health volunteers and patients with schizophrenia | BMJ Open

Simulation results using PK parameters obtained from PopPK analysis... | Download Scientific Diagram

Combining “Bottom‐up” and “Top‐down” Approaches to Assess the Impact of Food and Gastric pH on Pictilisib (GDC‐0941) Pharmacokinetics - Lu - 2017 - CPT: Pharmacometrics & Systems Pharmacology - Wiley Online Library