Teva Initiates Voluntary Nationwide Recall of One Lot of Topotecan Injection 4 mg/4 mL (1 mg/mL) Due to Presence of Particulate Matter | FDA
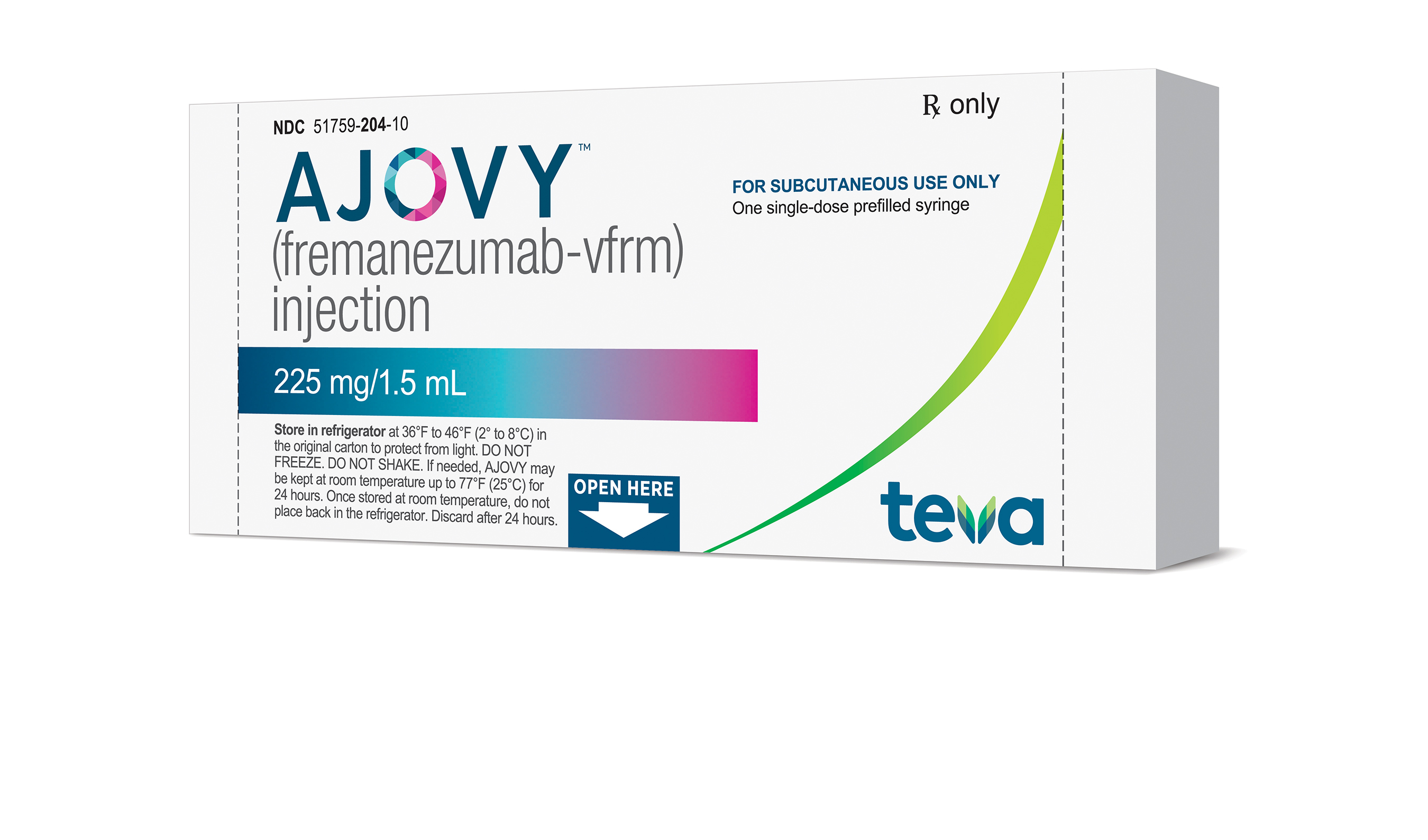
ADDING MULTIMEDIA Teva Announces U.S. Approval of AJOVYTM (fremanezumab-vfrm) Injection, the First and Only Anti-CGRP Treatment with Both Quarterly and Monthly Dosing for the Preventive Treatment of Migraine in Adults | Business
Teva Issues Recall of Tainted Blood Pressure Medicine, the Latest Global Recall for Valsartan | BioSpace

Teva Pharmaceuticals USA Issues Voluntary Nationwide Recall of all Amlodipine/Valsartan Combination Tablets and Amlodipine/Valsartan/Hydrochlorothiazide Combination Tablets that are Within Expiry | FDA
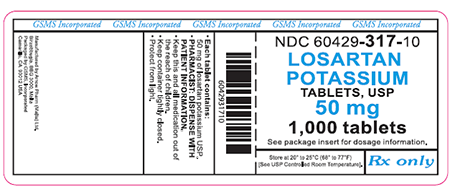
Teva Pharmaceuticals USA, Inc. Expands Voluntary Nationwide Recall of Losartan Potassium to 50 mg and 100 mg Tablets USP, Sold Exclusively to Golden State Medical Supply, Inc. | FDA